Determine which statements apply to hemoglobin myoglobin or neither – This comprehensive analysis delves into the intricacies of hemoglobin and myoglobin, exploring their molecular structures, functions, and physiological significance. By examining the similarities and differences between these two oxygen-binding proteins, we can determine which statements apply specifically to hemoglobin, myoglobin, or neither.
The chemical composition, oxygen-binding capacity, and allosteric regulation of hemoglobin and myoglobin will be thoroughly investigated, providing a comprehensive understanding of their distinct roles in the body.
Molecular Structure
Hemoglobin is a complex protein composed of four polypeptide chains: two alpha chains and two beta chains. Each chain is folded into a compact globular domain, and the four chains are arranged in a tetrahedral quaternary structure. The heme group, which contains an iron ion, is located in a pocket formed by each of the polypeptide chains.
Myoglobin is a smaller protein composed of a single polypeptide chain. The chain is folded into a compact globular domain, and the heme group is located in a pocket formed by the polypeptide chain.
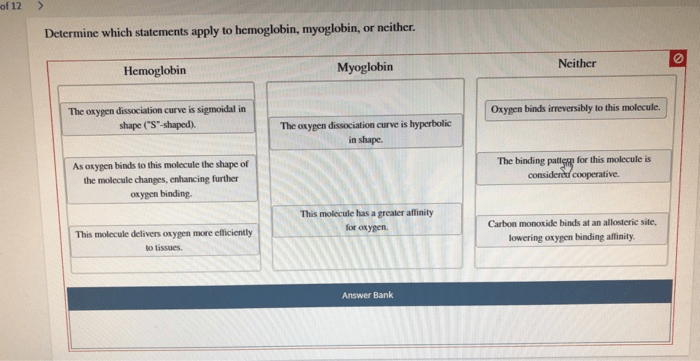
The molecular structures of hemoglobin and myoglobin are similar in many ways. Both proteins have a heme group that binds oxygen, and both proteins have a compact globular structure. However, there are some important differences between the two proteins. Hemoglobin is a tetramer, while myoglobin is a monomer.
Hemoglobin has a higher oxygen-binding capacity than myoglobin, and hemoglobin is allosterically regulated, while myoglobin is not.
Function

The primary function of hemoglobin is to transport oxygen from the lungs to the tissues. Hemoglobin binds oxygen in the lungs, where the partial pressure of oxygen is high, and releases oxygen in the tissues, where the partial pressure of oxygen is low.
The primary function of myoglobin is to store oxygen in muscle tissue. Myoglobin binds oxygen when the partial pressure of oxygen is high, and releases oxygen when the partial pressure of oxygen is low.
The functions of hemoglobin and myoglobin are similar in that both proteins bind and release oxygen. However, there are some important differences between the two proteins. Hemoglobin transports oxygen over long distances, while myoglobin stores oxygen over short distances. Hemoglobin is allosterically regulated, while myoglobin is not.
Oxygen Binding
Hemoglobin has a higher oxygen-binding capacity than myoglobin. This is because hemoglobin has four heme groups, while myoglobin has only one heme group. The oxygen-binding capacity of hemoglobin is also affected by the partial pressure of oxygen. At high partial pressures of oxygen, hemoglobin is saturated with oxygen.
At low partial pressures of oxygen, hemoglobin releases oxygen.
Myoglobin has a lower oxygen-binding capacity than hemoglobin. This is because myoglobin has only one heme group. The oxygen-binding capacity of myoglobin is also affected by the partial pressure of oxygen. However, myoglobin is not as sensitive to changes in the partial pressure of oxygen as hemoglobin.
The oxygen-binding properties of hemoglobin and myoglobin are different because of the different roles that these proteins play in oxygen transport and storage.
Allosteric Regulation
Hemoglobin is allosterically regulated, while myoglobin is not. Allosteric regulation is a process in which the binding of a molecule to one site on a protein affects the binding of a molecule to another site on the protein. In the case of hemoglobin, the binding of oxygen to one heme group increases the affinity of the other heme groups for oxygen.
Myoglobin is not allosterically regulated. This means that the binding of oxygen to one heme group does not affect the binding of oxygen to the other heme groups.
The allosteric regulation of hemoglobin is important for the efficient transport of oxygen in the body. The allosteric regulation of hemoglobin ensures that oxygen is released in the tissues, where it is needed.
Quaternary Structure
| Hemoglobin | Myoglobin | |
|---|---|---|
| Number of subunits | 4 | 1 |
| Molecular weight | 64,500 | 17,000 |
| Overall shape | Tetrahedral | Globular |
Location and Distribution: Determine Which Statements Apply To Hemoglobin Myoglobin Or Neither
Hemoglobin is found in red blood cells. Red blood cells are produced in the bone marrow and circulate throughout the body. Hemoglobin is also found in small amounts in muscle tissue.
Myoglobin is found in muscle tissue. Myoglobin is located in the cytoplasm of muscle cells.
The different locations of hemoglobin and myoglobin reflect the different roles that these proteins play in oxygen transport and storage.
FAQ
What is the primary function of hemoglobin?
Hemoglobin’s primary function is to transport oxygen from the lungs to various tissues throughout the body.
How does the molecular structure of hemoglobin differ from that of myoglobin?
Hemoglobin is a tetrameric protein, composed of four subunits, while myoglobin is a monomeric protein, consisting of a single subunit.
Does myoglobin exhibit allosteric regulation?
No, myoglobin does not exhibit allosteric regulation, unlike hemoglobin.