Embarking on a quest to identify the true statements about surface tension, we delve into a realm where the interplay of molecular forces and interfacial phenomena captivates the scientific mind. This exploration unveils the profound significance of surface tension in shaping the behavior of liquids, influencing diverse aspects of our world, from the mundane to the extraordinary.
Definition of Surface Tension
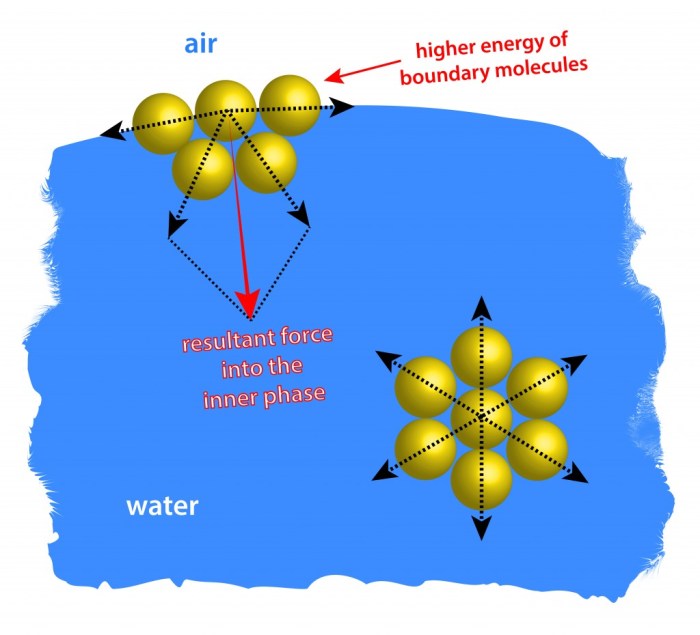
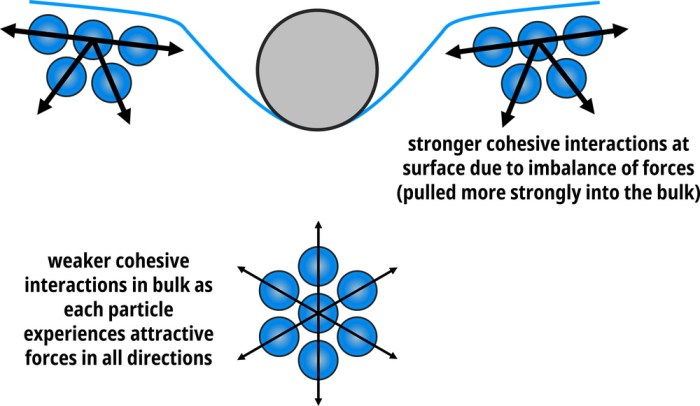
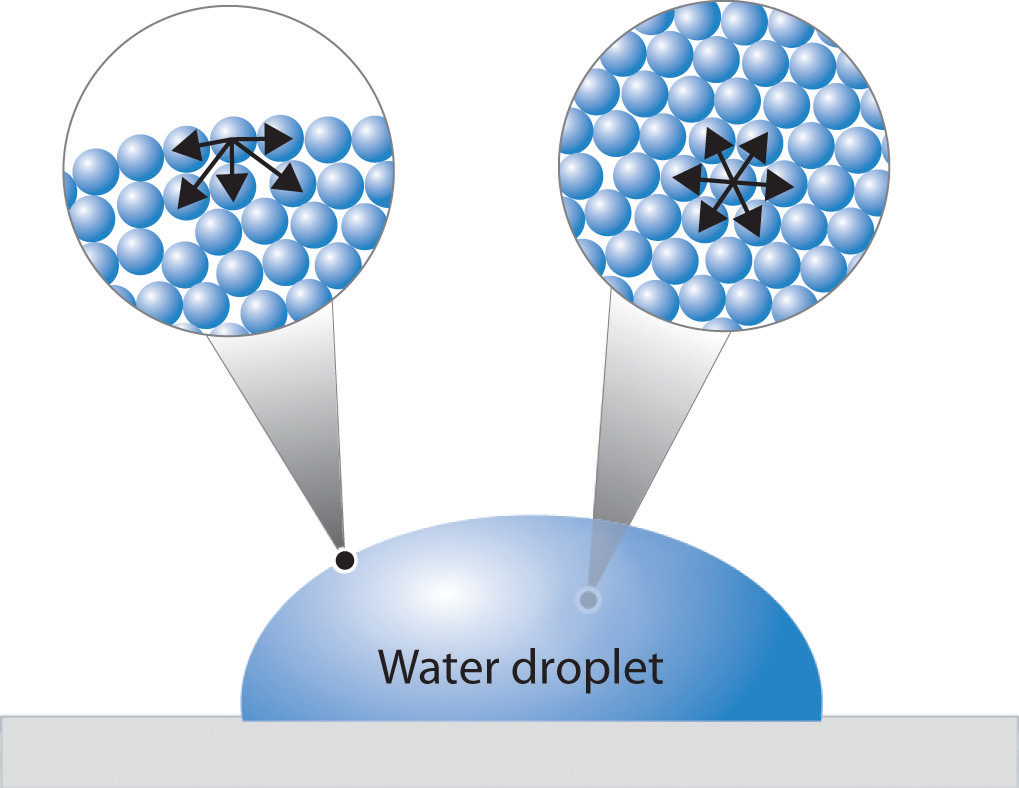
Surface tension is a physical phenomenon that occurs at the interface between two immiscible fluids, such as air and water. It is defined as the force acting on the surface of a liquid that tends to minimize its surface area.
This force is caused by the cohesive forces between the molecules of the liquid, which tend to draw them together and reduce the surface area.
Surface tension has a significant impact on the behavior of liquids. It is responsible for the formation of spherical droplets, the rise of liquids in capillary tubes, and the ability of insects to walk on water. It also plays a role in a variety of industrial processes, such as painting, coating, and printing.
Factors Affecting Surface Tension
Several factors can affect the surface tension of a liquid, including temperature, impurities, and the presence of surfactants.
Temperature
As the temperature of a liquid increases, the surface tension decreases. This is because the increased thermal energy causes the molecules of the liquid to move more rapidly and break free from the cohesive forces that hold them together.
Impurities
The presence of impurities in a liquid can also affect its surface tension. Impurities can either increase or decrease the surface tension, depending on their nature. For example, the addition of salt to water increases the surface tension, while the addition of soap decreases the surface tension.
Surfactants
Surfactants are substances that can reduce the surface tension of a liquid. They are often used in detergents, wetting agents, and emulsifiers. Surfactants work by adsorbing to the surface of the liquid and disrupting the cohesive forces between the molecules.
Measurement of Surface Tension
There are several methods that can be used to measure the surface tension of a liquid.
Capillary Rise Method
The capillary rise method is a simple and inexpensive method for measuring surface tension. It involves measuring the height to which a liquid rises in a capillary tube. The surface tension can then be calculated using the following equation:
“`γ = ρghr“`where:
- γ is the surface tension
- ρ is the density of the liquid
- g is the acceleration due to gravity
- h is the height of the liquid in the capillary tube
- r is the radius of the capillary tube
Pendant Drop Method
The pendant drop method is a more accurate method for measuring surface tension. It involves measuring the shape of a drop of liquid suspended from a needle. The surface tension can then be calculated using the following equation:
“`γ = Δρgr^2/2“`where:
- γ is the surface tension
- Δρ is the difference in density between the liquid and the surrounding gas
- g is the acceleration due to gravity
- r is the radius of the drop
Applications of Surface Tension, Identify the true statements about surface tension
Surface tension has a wide range of applications in industry and biology.
Industrial Applications
Surface tension is used in a variety of industrial processes, including:
- Painting: Surface tension is used to control the flow of paint and prevent it from dripping or running.
- Coating: Surface tension is used to control the thickness and uniformity of coatings.
- Printing: Surface tension is used to control the transfer of ink from the printing plate to the paper.
Biological Applications
Surface tension also plays an important role in a variety of biological processes, including:
- Respiration: Surface tension helps to keep the alveoli in the lungs open.
- Circulation: Surface tension helps to keep the blood vessels open.
- Cell division: Surface tension helps to divide cells.
Frequently Asked Questions: Identify The True Statements About Surface Tension
What is surface tension?
Surface tension is the force that causes the surface of a liquid to behave like a stretched elastic membrane. It arises from the cohesive forces between liquid molecules, which tend to minimize the surface area of the liquid.
How does temperature affect surface tension?
Generally, increasing temperature decreases surface tension. This is because higher temperatures increase the kinetic energy of the molecules, making them less cohesive and reducing their tendency to form a tightly bound surface.
What are some applications of surface tension?
Surface tension finds applications in various fields, including:
- Detergents and surfactants: Surface tension plays a crucial role in the effectiveness of detergents and surfactants, which reduce surface tension to enhance cleaning and wetting properties.
- Inkjet printing: Surface tension governs the formation and ejection of ink droplets in inkjet printing technology.
- Biological systems: Surface tension influences cell adhesion, capillary action in plants, and the formation of lipid bilayers in cell membranes.